Flocculants: what they are and how they differ from coagulants
p> Flocculants are reagents that separate impurities from water. They complement coagulants - they bind impurities into larger and denser flocculus flakes. As a result, no suspended solids remain in the water - it becomes more transparent, and the sediment can be easily separated by clarifiers, settling tanks, filters and other special equipment.
A solution of flocculants added to the liquid helps separate it into water and impurities faster. Therefore, flocculants are often used for water treatment.
How flocculants work
Flocculants are bead-like macromolecules. Several pollutant particles adhere to them at the same time, and flocculi are produced.
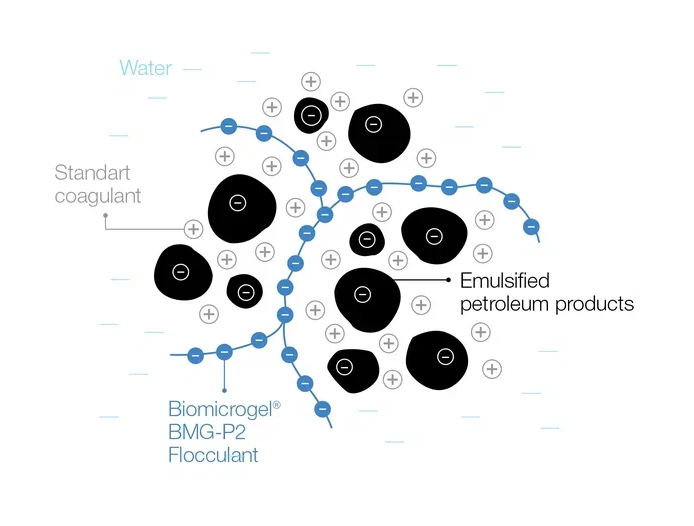
Separation of a water-oil emulsion: the coagulant groups the petroleum product particles, the flocculant connects them by polymer bridges and forms larger groups
Flocculants are added to the liquid after the coagulants in order to thicken and compact the sludge that results from coagulation. But in some cases, they can be used without coagulants and first collect impurities and then form flocculates from them.
Differences between flocculation and coagulation
Flocculation is a type of coagulation. It, too, groups fine suspended particles and forms a sediment, but there are differences between the processes:
-
Principle of operation. Coagulants remove the charge from the particles, and they can already collide with each other to form groups. Flocculants connect these groups with polymer bridges.
-
Reagents used. Metal salts are used for coagulation: iron chloride, iron sulfate, aluminum chloride, aluminum sulfate and others. In flocculation mainly organic substances are used: anionic, cationic, amphoteric flocculants based on polyacrylamide, acrylate and other substances.
-
Number of reagents. Coagulation requires ten times more reagents than flocculation. If 20 mg of coagulant is needed per liter of liquid, flocculant requires 1-2 mg.
-
Sludge volume. Flocculation produces a larger sludge than coagulation and is easier to separate from the liquid.
Advantages and disadvantages
Since flocculation and coagulation are similar processes, they have advantages in common. The disadvantage, too, is the use of reagents.
Advantages:
-
High degree of water purification and the ability to control the degree of purification according to the required indicators.
-
Versatility. Both processes are applicable to any volume of water.
-
They do not require special equipment, work with any filters, and do not shorten their service intervals. Flocculants also save coagulants consumption.
Treatment of low-colored water with flocculants saves 10-40% of coagulant consumption and makes water more transparent.
When comparing coagulation and flocculation, the advantage of the latter is a more compact, dense sludge and consequently more transparent water.
This advantage allows:
-
less frequent maintenance and repair of the filtration system because there are no small particles in the water that clog the filter.
-
Filter out coarse sediment faster, saving time on processes that reduce water color and turbidity. This helps treat more liquid.
Types of flocculants
According to their composition, they can be divided into two large groups - organic and inorganic. Organic ones are mainly used, of the inorganic ones, only silicic acid is used.
According to their origin, organic ones are divided into natural and synthetic. The latter are more toxic, but cheaper.
Natural and synthetic reagents are ionic and non-ionic according to the presence of charge. Nonionic flocculants have no charge and are suitable for cleaning water with uncharged particles. Reagents with an electric charge - ionic - interact with charged particles.
By the sign of charge, an ionic flocculant can be:
-
Anionic - negatively charged. Interacts with inorganic compounds.
-
Cationic - positively charged. Suitable for cleaning water from organic suspended solids.
-
Amphoteric - exhibits cationic, anionic or neutral properties depending on liquid pH: in acidic environment it behaves as cationic, in alkaline one - as anionic, in equilibrium one - as nonionic.
An example of a naturally occurring organic flocculant is Biomicrogels® BMG-C2, a solution derived from recycled plant material
Flocculants are available in granule, powder, paste, gel or solution form.

Biomicrogels® comes in flocculant BMG-X2 as a powder in a bag and BMG-C200.5 as a solution in a can, barrel or IBC-container
Where flocculants are used
They are used wherever fine particles need to be concentrated into larger groups. The target of this process can be either water or particles themselves.
In agriculture, they strengthen soils which are made up of dust particles and retain water in them. In the mining and processing industry, the use of flocculants helps isolate more of the mined substance from the pulp: coal, copper, iron, and more.
- Plants to clean bottom and by-product water or industrial effluent. The use of flocculants helps purify water to levels at which it can be discharged to treatment plants without harm to the environment or returned to the production cycle.
-
Water treatment plants, where chemicals are added to the water before settling tanks and clarifiers. They speed up the formation and sedimentation of flakes and make the water clearer. Flocculants help when a plant does not have enough capacity to treat more water.
-
A wastewater treatment plant where flocculants dewater the solid sludge that remains after wastewater treatment. As a result, the sludge drying area takes up less space at the plant.
-
In swimming pools, to purify water in a way that is safe for human health. Flocculus is removed with a net or vacuum cleaner.
Conclusion
Flocculants enhance the effect of coagulants: they speed up the separation of impurities from the water, form a larger and denser sludge, and save the consumption of coagulants. They extend the life of filters and help water treatment plants treat more fluids. The only disadvantage of coagulation and flocculation - the use of reagents - is solved by choosing reagents of natural origin.




